Transforming Pharmaceutical Inspections
Transforming Pharmaceutical Inspections
In the pharmaceutical industry, compliance with stringent regulatory standards is crucial for ensuring the safety and efficacy of products. Regulatory bodies like the FDA, EMA, and others enforce rigorous guidelines that govern everything from manufacturing processes to quality control measures. A key aspect of maintaining compliance is regular inspections, which are essential for verifying adherence to these standards. Aaseya is at the forefront of providing cutting-edge inspection solutions that not only streamline the inspection process but also enhance operational efficiency and ensure continuous compliance.
The Need for Advanced Inspection Solutions
The conventional ways of inspecting control processes in pharmaceutical industry quite frequently can be characterized by recurrent instance of shifting emphasis from one stage of the process to the other, data collection using pen and paper, as well as procedures that tend to consume so much time. Such methods could give rise to quite a few problems including but not limited to:
1. Data Collections That Are Once Biased: Some processes are manual hence complete via movement, Harrison Neshe is a case in point laying the risks of data collection and once it is replicated so is the data reconciliation and reporting.”
2. Low Productivity: Use of paper systems however is so slow because of the inability to dispose or import and export data on a management level, and in most instances, it hinders efficient inspection data management.
3. Slow Action: Absence of data that is available instantly effective action can be difficult and in the end effect compliance issues in a sufficiently timely manner cannot be achieved.
4. Compliance Issues: Compliance issues often arise when there’s a lack of clarity in understanding the boundaries of current systems. This, coupled with the constantly changing and cumbersome regulatory processes, makes compliance a challenging task.
Potential ways to Transform Pharmaceutical Inspections:
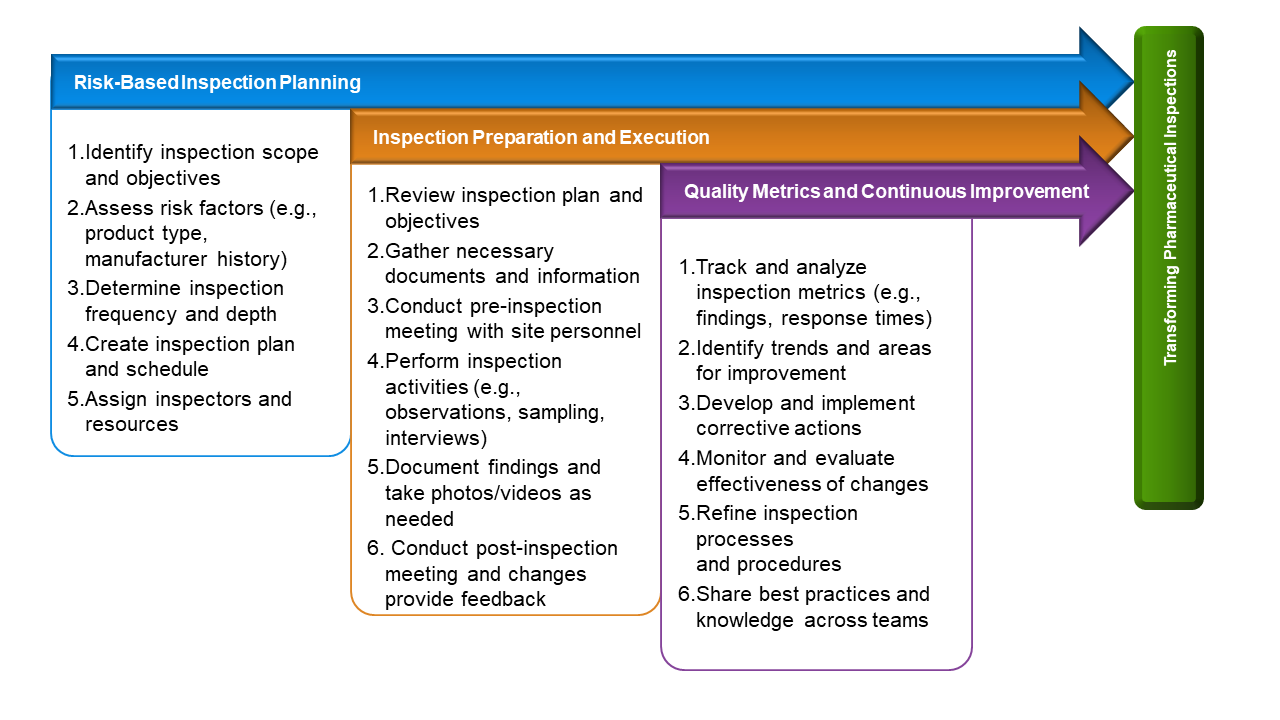
Aaseya’s Inspection Solution: A Game-Changer for Compliance and Efficiency
Our innovative inspection solution is designed to transform the way pharmaceutical companies conduct inspections, ensuring accuracy, efficiency, and compliance. Here’s how:
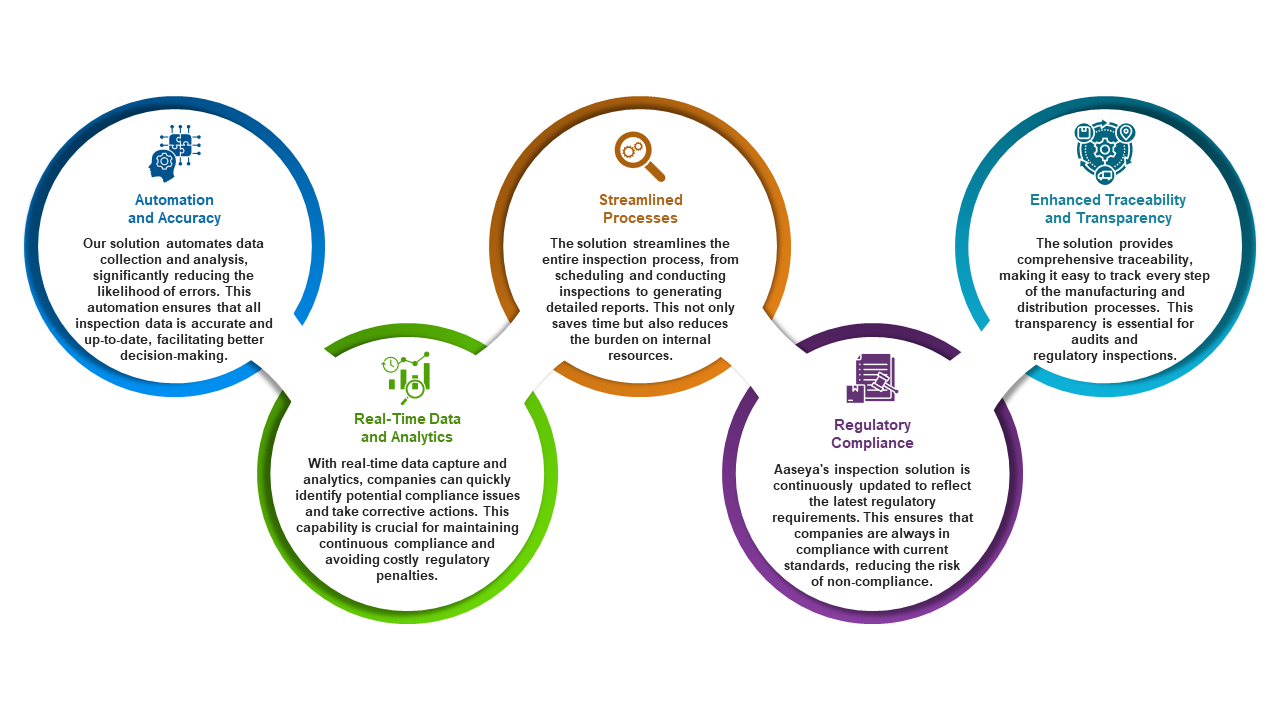
Success Story: Aaseya’s Solution in Action
One of our clients, a leading pharmaceutical manufacturer, faced significant challenges with their traditional inspection processes. The manual nature of their system led to frequent data inaccuracies, delays in reporting, and difficulties in keeping up with regulatory changes. After implementing Aaseya’s inspection solution, they experienced remarkable improvements:
- Reduction in Inspection Time: Automated processes reduced the time spent on inspections by 50%, allowing the company to focus more on core operations.
- Improved Data Accuracy: The automation of data collection minimized errors, ensuring that all records were accurate and reliable.
- Enhanced Compliance: Real-time analytics and updates helped the company stay compliant with the latest regulations, avoiding potential fines and penalties.
- Increased Operational Efficiency: The streamlined inspection process freed up valuable resources, which were then reallocated to other critical areas of the business.
Looking Ahead: The Future of Pharmaceutical Inspections
The pharmaceutical industry is undergoing constant changes, and so are the methods for ensuring compliance and quality. Aaseya is committed to remaining on the front line of inspection solutions by consistently improving our services according to the changing demands of clients. We aim to provide pharmaceutical companies with what they need for maintaining their highest quality and safety levels, in addition to improving operation efficiency and cost reduction.
Invest in Aaseya’s inspection solution today, taking a milestone towards streamlining an obedient, effective, triumphant pharmaceutical environment. In unity, we can ascertain the security and healthiness of customers globally.
Conclusion:
Compliance issues often arise when there’s a lack of clarity in understanding the boundaries of current systems. This, combined with the ever-changing and complex regulatory landscape, makes it increasingly difficult for organizations to maintain compliance, manage risks effectively, and avoid costly penalties.
